Als New Treatment 2025
Als New Treatment 2025. Treatment with neurosense therapeutics’ primec was found to extend patients’ time without complications or death, and to lead to clinically meaningful effects on quality of life, among those with amyotrophic lateral sclerosis (als). Spinogenix announces fda clearance of ind application for spg302, a novel therapy for the treatment of als.
A major breakthrough in the treatment of amyotrophic lateral sclerosis, known as als, can potentially help stop the disease in its tracks in as much as half of. A new path for als treatment.
Congress makes critical decisions about the future of als research through the annual appropriations process.

New ALS treatment Immunotherapy with T cells, Projenx will conduct a phase 1c study of a new drug called prosetin that was designed to protect motor neurons from the destructive processes triggered by als. Our top advocacy priority each year aims to increase federal.
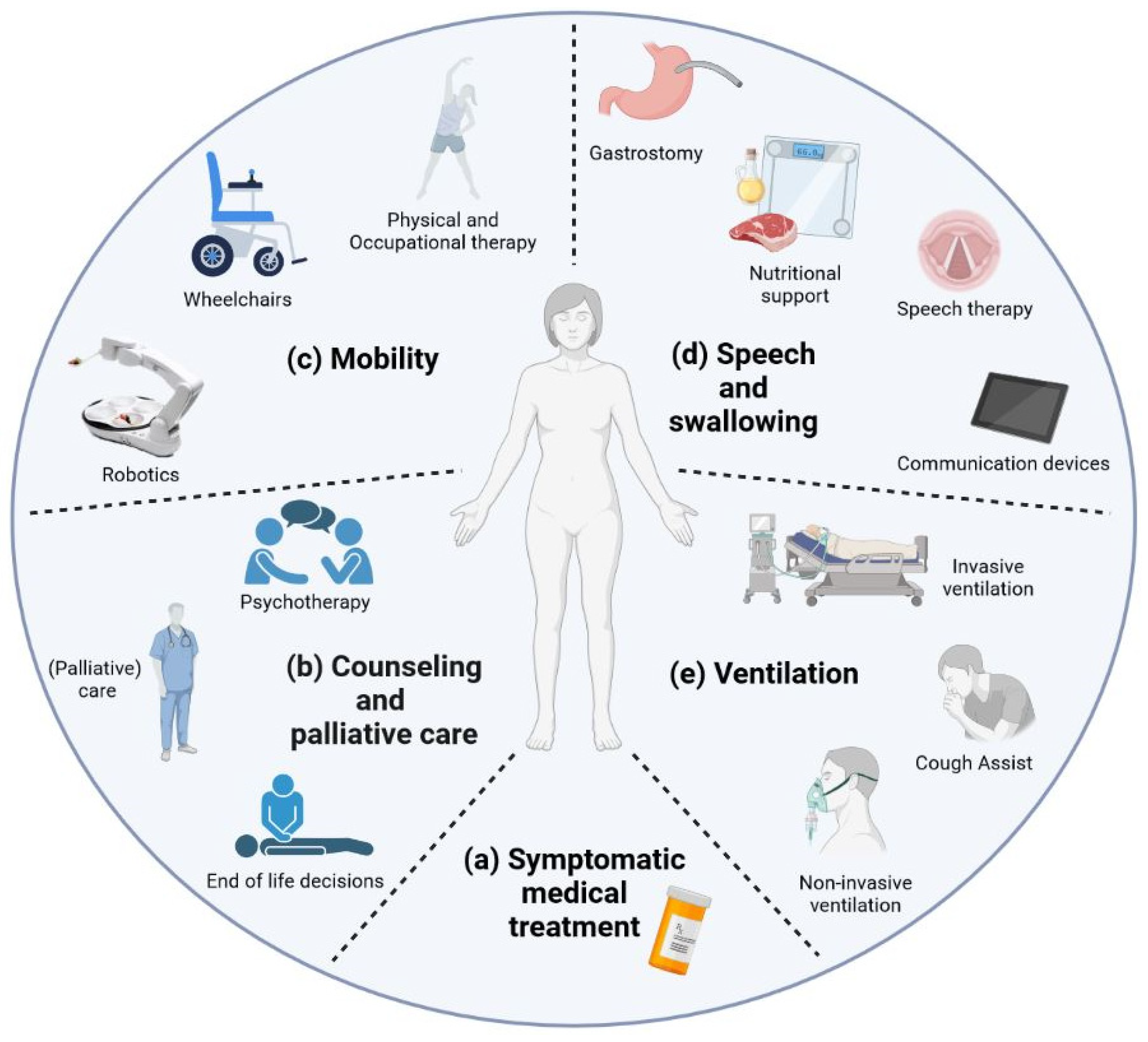
Cells Free FullText Current State and Future Directions in the, By mary chapman november 7, 2023. To date, treatment options for als remain severely limited, have modest benefits, and no known treatment halts or reverses the progression of als.
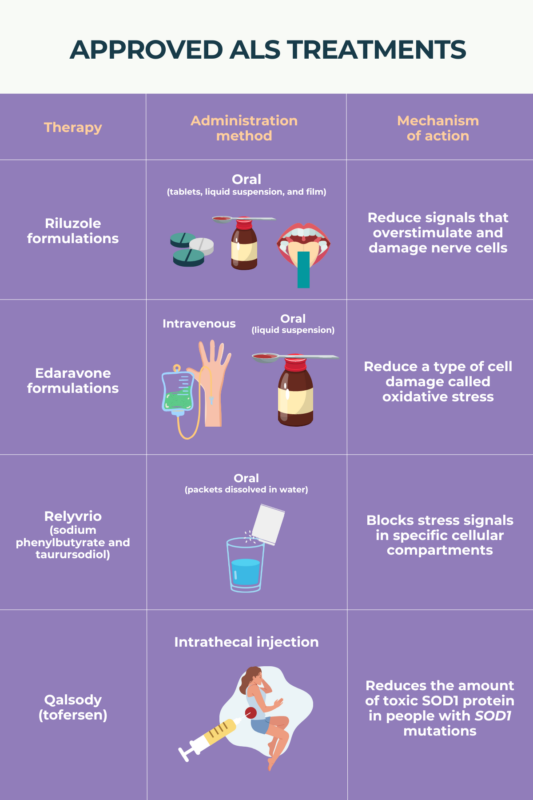
Amyotrophic Lateral Sclerosis (ALS) Treatment ALS News Today, New research out of london, ont.'s western university is shedding light on a potential cure for als, with the goal of conducting human clinical trials within the next. In the quest to find a cure for als patients, a team of researchers led by usc stem cell scientist justin ichida has identified two promising avenues for.

Muscular Dystrophy Association Celebrates FDA Approval of Biogen’s, The committee recommended granting a. Ema’s human medicines committee (chmp) recommended ten medicines for approval at its june 2025 meeting.
New ALS treatment Immunotherapy with T cells, Treatment with neurosense therapeutics’ primec was found to extend patients’ time without complications or death, and to lead to clinically meaningful effects on quality of life, among those with amyotrophic lateral sclerosis (als). To date, treatment options for als remain severely limited, have modest benefits, and no known treatment halts or reverses the progression of als.
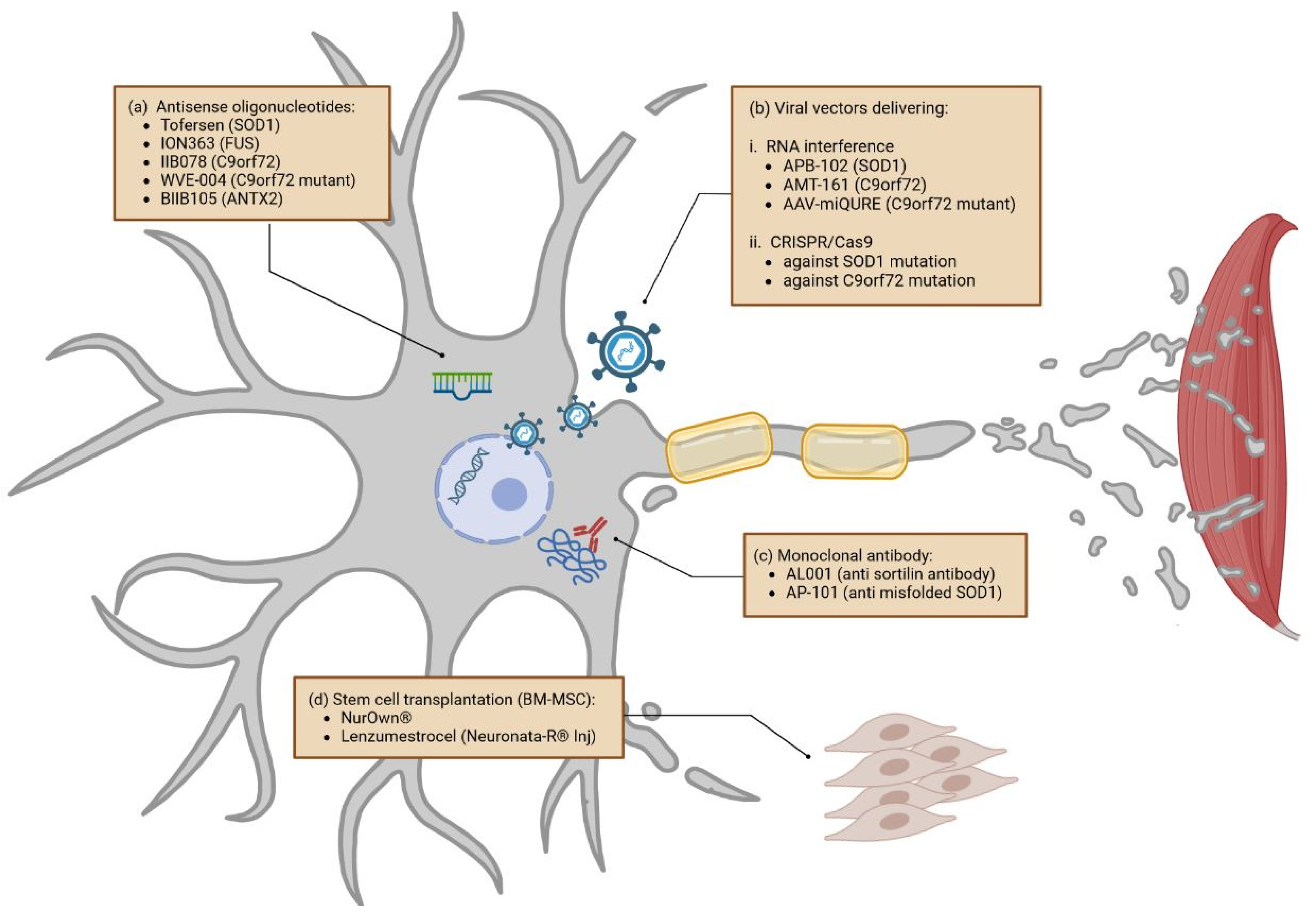
Cells Free FullText Current State and Future Directions in the, The biotechnology company projenx recently. Our top advocacy priority each year aims to increase federal.

Regenerative Medicine A New Path for ALS Treatment CedarsSinai, Relyvrio (sodium phenylbutyrate/taurursodiol) is the first new als treatment to receive fda approval since 2017. Even though curative treatment options, able to prevent or stop disease progression, are still unknown, recent breakthroughs, especially in the field of targeting.

New drug to treat ALS now available in the U.S., A major breakthrough in the treatment of amyotrophic lateral sclerosis, known as als, can potentially help stop the disease in its tracks in as much as half of. A new treatment shows promise against the deadly neurodegenerative disease als, a study based on mice showed tuesday.

ALS Association Urges FDA to Approve New ALS Treatment Just Approved in, Michael strong has uncovered a potential path toward. Even though curative treatment options, able to prevent or stop disease progression, are still unknown, recent breakthroughs, especially in the field of targeting.
4 Treatments That Can Improve Life With ALS ALS News Today, Our top advocacy priority each year aims to increase federal. A new treatment shows promise against the deadly neurodegenerative disease als, a study based on mice showed tuesday.
Congress makes critical decisions about the future of als research through the annual appropriations process.